Agriculture - Fertilisers
 The need for fertilisers:
The need for fertilisers:
Green plants photosynthesise and manufacture carbohydrates, proteins, lipids and other organic compounds. Some of these molecules require the plant to absorb inorganic minerals in order to make these compounds. These are absorbed from the soil in solution.
Farmers harvest crops year after year and export these minerals away from the field which creates an in-balance in some soil nutrients. In order to continue to produce high yields and quality crops, a farmer will have to artificially add fertilisers.
Types of fertiliser:
- Natural organic fertilisers – e.g. manure.
Faeces from farm animals is collected and then allowed to decay for several months. This is then spread onto fields. It is usually applied to grass as its nutrient content (Nitrogen, Phosphorous and Potassium) content is variable and unknown and unsuitable for cereal crops.
- Artificial inorganic fertilisers – e.g. compound granules with exact levels of NPK.
These are manufactured industrially and each crop will have a suitable blend of the major nutrients (NPK) and additions of other minerals (micronutrients) in less quantities.
Hazards of using fertilisers:
- Over centuries, farmers have ‘manured’ their fields by adding organic fertiliser or ‘muck’. It also has the advantage of removing large quantities of farm animal waste by recycling (Nitrogen and carbon cycles)
- Modern high yielding crops require inorganic fertilisers in order to gain high yields and quality .
- Farmers can go on adding fertilisers to their crops and there will be a steady rise in the yield until a level is reached, where there is no further increase.
 There is an optimum level of fertilizer to be added, beyond which the law of diminishing returns. Above a certain amount of fertilizer there is no further increase in yield, in fact the yield may even go down.
There is an optimum level of fertilizer to be added, beyond which the law of diminishing returns. Above a certain amount of fertilizer there is no further increase in yield, in fact the yield may even go down.- If too much fertiliser is added to the soil (especially if the weather is very wet), the plants cannot absorb it and it will leach (wash out) of the soil and enter waterways.
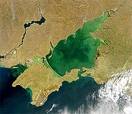
- This leads to the ‘Eutrophication’ of rivers, ponds and lakes.

Eutrophication
|
UNEP webpages on  How bad is Eutrophication at present? How bad is Eutrophication at present?
• Serious damage by this form of water pollution has occurred many times and continues to be a problem in areas where there is intensive cereal growing.
• There are other non-farming activities than can cause eutrophication
|

|

 Pesticides
Pesticides




 The need for fertilisers:
The need for fertilisers: There is an optimum level of fertilizer to be added, beyond which the law of diminishing returns. Above a certain amount of fertilizer there is no further increase in yield, in fact the yield may even go down.
There is an optimum level of fertilizer to be added, beyond which the law of diminishing returns. Above a certain amount of fertilizer there is no further increase in yield, in fact the yield may even go down.
What's your opinion?
Average rating




Current rating: 5/5 (from 1 votes cast)